Synopsis
Prescription drugs have been a key driver of health care spending growth in the United States. In some countries, including Australia, drugs must pass a "fourth-hurdle" review of cost-effectiveness after they pass other rigorous tests. Such a process not only helps to ensure society is getting appropriate value for what it spends on drugs, but also helps to reward innovation. The U.S. may benefit from studying Australia’s experience and experimenting with comparative effectiveness review.
The Issue
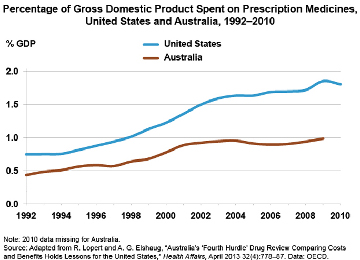
Health care makes up 18 percent of the U.S. gross domestic product—nearly twice the average of countries within the Organization for Economic Cooperation and Development (OECD). Prescription medicines are one of the key drivers of high U.S. costs, with drug spending increasing at up to twice the rate of health care spending overall. In a Health Affairs article, two former Commonwealth Fund Harkness Fellows highlight Australia’s fourth-hurdle drug review process as a possible model for the U.S. After testing new drugs for safety, efficacy, and quality, the Australian government assesses for value, and makes coverage decisions accordingly.
How Australia’s Drug Review Process Works
Australians enjoy universal coverage of prescription drugs. Patients contribute fixed copayments based on income, and annual outlays are capped to protect individuals from catastrophic costs. Faced with high prices and rising costs in the late 1980s, the government phased in its fourth-hurdle process, with cost-effectiveness becoming a mandatory prerequisite for formulary listing in 1993. If a proposed drug is substantially more costly than available alternatives, it may be included in the formulary only if it offers a clinical advantage to some patients.
Impact on Spending, Value, and Innovation
While the Australian approach does not prevent spending growth, it does ensure that the opportunity costs of adding new drugs are identified. "The approach acknowledges that resources are inevitably finite, even when programs are uncapped, so that if a low-value product is funded, some higher-value product or program may be crowded out," the authors say. "At a macro level, this is a fundamental issue for the U.S., because unconstrained growth in health expenditure effectively crowds out other key programs in education and infrastructure."
The fourth-hurdle system, the authors say, rewards true innovation by indirectly focusing financial incentives toward products that can make a significant impact on health outcomes, while subtly discouraging those that do not. The system does not try to ration high-value medicine. Instead, it supports their addition to the formulary, while identifying drugs that offer only marginal health gains and either excluding them or negotiating prices proportionate to their benefit.
What Does This Mean for the United States?
In the U.S., the importance of addressing value in health care purchasing is rarely acknowledged. Instead, rising prices are absorbed in the form of higher premiums, coverage exclusions or limits, and increasing copayments. However, a recent survey of oncologists found that 80 percent favored greater use of cost-effectiveness data in insurance coverage decisions. In addition, the American College of Physicians, Veterans Health Administration, and Medicare Payment Advisory Commission have all affirmed the need for information on comparative effectiveness.
Moreover, the decentralized, fragmented nature of health care in the U.S. is not necessarily a barrier to implementation, the authors say. Individual payers, for example, could institute their own fourth-hurdle drug review programs. Still, "an arrangement in which several payers shared centralized 'back office' review processes might be more feasible and could have the added benefit of providing cooperative strength in purchasing power," they conclude.
The Bottom Line
Australia’s experience illustrates that comparative effectiveness review in prescription drug coverage is both feasible and useful in identifying value. In the United States, transparent, evidence-based decision-making could help bend the cost curve and improve health outcomes for many.