Earlier this month, President Biden signed an executive order, Promoting Competition in the American Economy. The order lays out actions to address consolidation in health care markets and price competition in the prescription drug markets. The administration has indicated that lowering drug prices is a priority and instructed agencies to act. In addition, Congress is working to move drug pricing reform this year. A comprehensive approach is needed that focuses on lowering launch prices and limiting price increases, encouraging price competition through generics and biosimilars, eliminating anticompetitive behaviors of brand-name manufacturers, reforming drug patent processes, and addressing misaligned incentives in Medicare. This blog post examines the executive order’s call for the Centers for Medicare and Medicaid Services (CMS) to consider payment models to control spending and lower costs for prescription drugs and outlines the approaches CMS might consider in the Medicare program.
What actions does the executive order take to lower prescription drug pricing?
- Supports the legislative reforms being considered by Congress: allowing Medicare to negotiate drug prices, redesigning Part D to reduce costs for beneficiaries and taxpayers, and implementing an inflation-based rebate penalty for excessive price increases.
- Continues previous activities such as: the Trump administration’s generic and biosimilar action plans for competition, allowing states and Tribes to import drugs from Canada, and shoring up the drug supply chain.
- Outlines new responsibilities to agencies, including instructing the Food and Drug Administration, along with the Federal Trade Commission and Patent and Trademark Office, to identify and address anticompetitive behaviors that impede generic and biosimilar competition. CMS is tasked with exploring payment models to increase utilization of generic drugs and biosimilars.
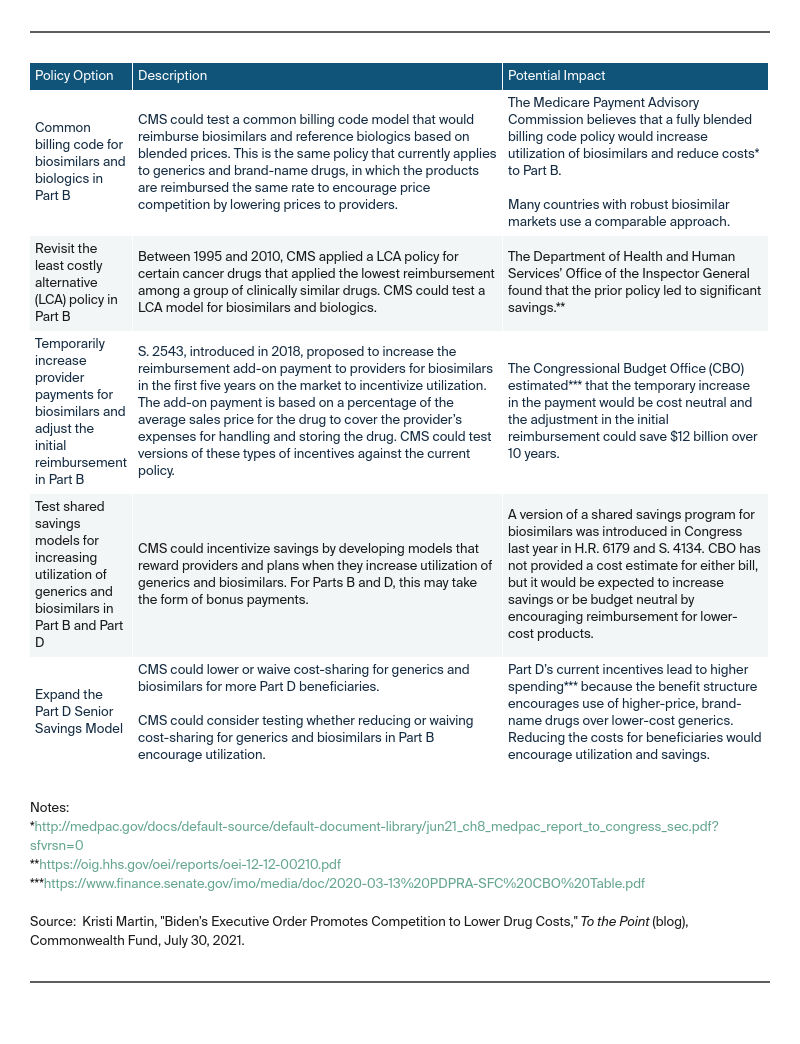
To date, what has CMS done to encourage generic and biosimilar competition?
Over the last several years, CMS has proposed demonstrations to lower costs by encouraging the use of generic1 and biosimilar products,2 although to date they have not lowered costs or increased utilization of generic or biosimilar drugs. In 2016, CMS implemented a Part B policy across Medicare to reimburse all competing biosimilars at the same reimbursement rate. Under this policy, biosimilars competed against one another, but not against the reference biologic. In 2018, CMS changed the policy to give each biosimilar its own billing code and reimburse providers an add-on payment based on the reference biologic. The impact of this policy change is unclear and there is debate over which policy encouraged more robust price competition between biosimilars and biologics. More recently, CMS launched the Part D Payment Modernization Model, a voluntary model to test changes to the Part D payment structure using performance-based payments based on increased utilization of generic and biosimilar drugs with lower list prices and reduced cost-sharing for low-income beneficiaries. Part D plan sponsors have shown little interest in participating.
Although these policy options have not had an impact in the short term, emerging research is showing that reimbursement policy changes can improve biosimilar uptake among providers.
What other reimbursement models could Medicare consider to increase utilization of generics and biosimilars and lower costs?
Under the executive order, CMS could explore testing policies under its Innovation Center authority with a goal of increasing generic and biosimilar utilization to lower costs to Medicare. These policy options should include education for providers, including clinical decision support tools, as well as safeguards to ensure access for beneficiaries, such as exceptions processes. The following table summarizes policy options CMS could take.