Background
Hopes are high that an effective vaccine against the novel coronavirus will normalize life and revitalize an economy upended by the COVID-19 pandemic. Based on strong initial trial results, the U.S. Food and Drug Administration (FDA) is poised to grant emergency authorization to the first of several vaccines before the end of 2020.1
To move new vaccines to authorization so quickly is an unprecedented scientific achievement, but it is merely a first step. Success in manufacturing, distributing, and administering the vaccine will depend on thoughtful planning and logistics, as well as on clear communications about the benefits and risks to the public.2 Until a substantial percentage of the population is vaccinated, the virus will continue to circulate and wreak havoc on the health of Americans and the economy.
Mounting a successful COVID-19 vaccination program in the United States will be especially challenging. States have substantial autonomy to chart their own course on public health, and historically they are responsible for organizing public health interventions in response to disease outbreaks.3 The federal government, under the leadership of the incoming Biden administration, will be tasked with ensuring effective and equitable vaccination across states through a coherent national strategy that provides allocation guidance, distribution infrastructure, and additional resources.
To better understand the challenges ahead and the potential variation in outcomes across states, this brief examines the U.S. COVID-19 pandemic experience to date and analyzes two prior vaccination programs: for seasonal influenza in 2019 and for the H1N1 flu pandemic from 2009 to 2010. Data for our analysis come from the Centers for Disease Control and Prevention’s (CDC) 2019 Behavioral Risk Factor Surveillance System (BRFSS) survey and from CDC analyses of multiple surveys from 2009–2010 (see “Data Sources” below for further detail).
Even though COVID-19 is more contagious and more lethal than influenza, experience with these prior vaccination efforts is still relevant. Both programs aimed to vaccinate more than 70 percent of all adults, on a voluntary basis, within a matter of months; this will likely be the goal for any COVID vaccine program as well. Another element in common is the limited lead time for both manufacturing and distribution. A new influenza vaccine is manufactured every year based on circulating virus detected during the prior year. In the case of H1N1 — a novel virus first identified six months prior to flu season in the Northern Hemisphere — the timeline for manufacturing and distribution was especially short.
Highlights
- The success of a future COVID-19 vaccination program rests on achieving high rates of uptake, especially in states with higher case counts and states with larger Black, Latino, and American Indian populations.
- The federal government’s strategy should aim for higher COVID-19 vaccine adherence than is typically achieved for influenza and ensure equitable availability across states. In 2019, adult seasonal flu vaccination rates in all U.S. states and the District of Columbia fell well below the population goal of 70 percent, with none achieving a rate above 51 percent. During the 2009–2010 H1N1 pandemic, uptake was even lower.
- Since the start of the pandemic, several states that have experienced large increases in COVID-19 cases have also in the past had lower flu and H1N1 vaccination rates.
- Nearly all states have reported significant racial and ethnic disparities in vaccination, suggesting the need for special efforts to reach communities at higher risk from COVID-19.
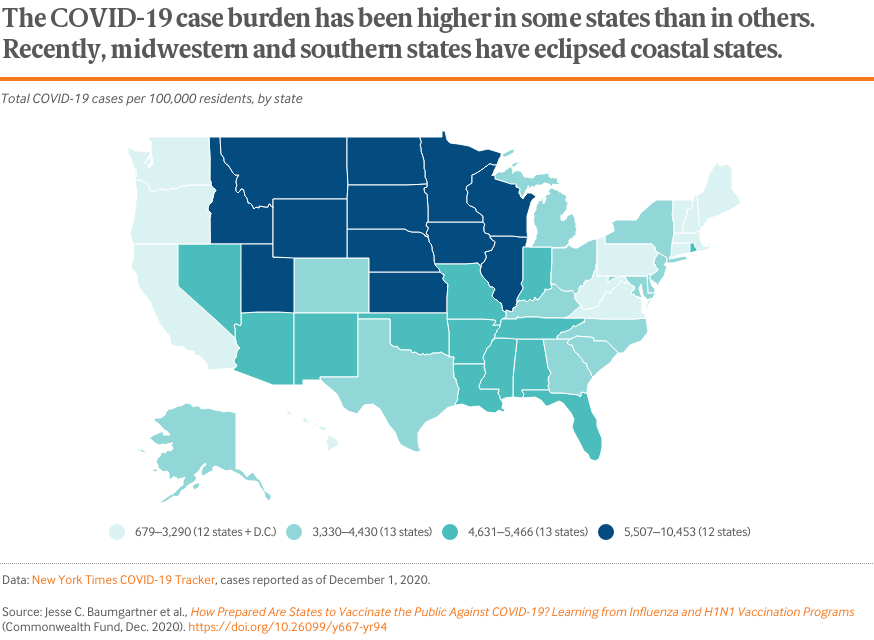
Findings
The COVID-19 pandemic is now active in every corner of the United States.4 Although the initial hotspots were in the Northeast — New York and New Jersey in particular — the virus has spread widely since then. Since the beginning of the pandemic, several southern states have experienced large sustained outbreaks, including Arizona, Florida, and Texas. Recently, many midwestern states have reported severe outbreaks and now have some of the highest numbers of cases per capita, especially Iowa, the Dakotas, and Wisconsin.
Experience to date suggests it may be difficult to predict which states will be at highest risk during the next year. Areas in the midst of outbreaks and with high levels of community transmission will benefit most once vaccines become available.
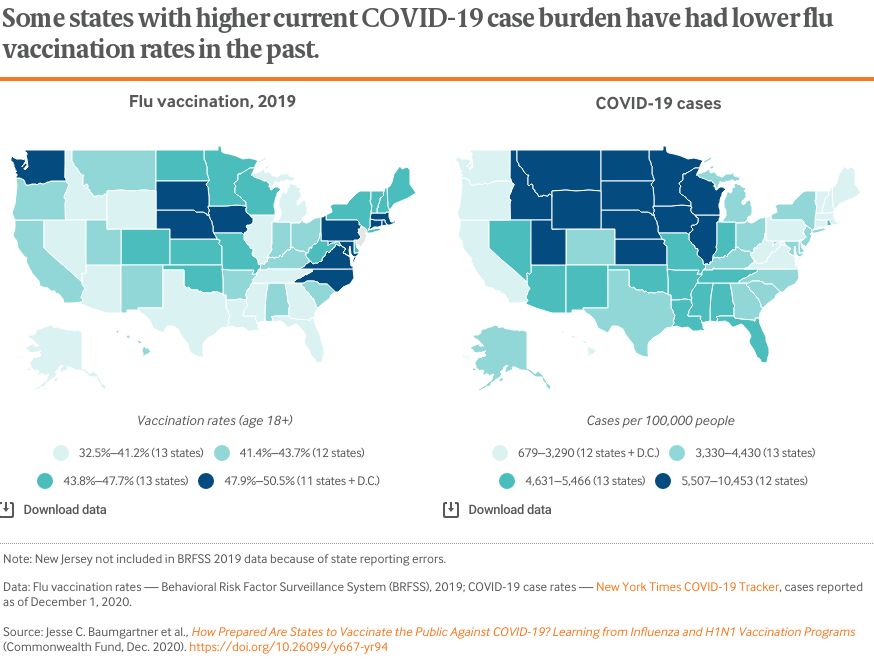
Adult flu vaccination rates, which largely depend on private distribution and administration, have historically been suboptimal.5 In calendar year 2019, the national rate for all adults age 18 and older was 43 percent, the highest in the past decade.6 That rate, however, was still well below the 70 percent population goal established by the federal government’s Healthy People 2030 campaign.7
Vaccination rates vary by as much as 50 percent across states, but no state achieved higher than 51 percent in 2019 (also see Table 1). Many states with high COVID-19 case levels also report some of the lower flu vaccination rates, including the Rocky Mountain and southern regions.
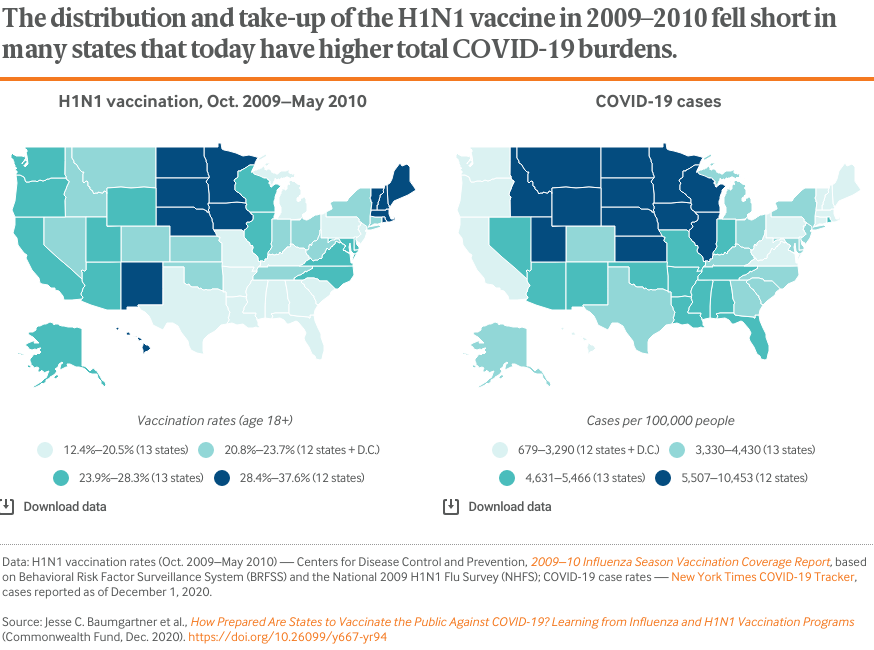
Although vaccine distribution and allocation during a pandemic is directed by states in close partnership with federal agencies, the adult uptake of the H1N1 vaccine from October 2009 through May 2010 was also low nationally (approximately 23%, partially because of early supply shortages; see Table 3). It exhibited similar regional patterns to the seasonal flu vaccine, including notably lower vaccination rates across the South (also see Table 1).
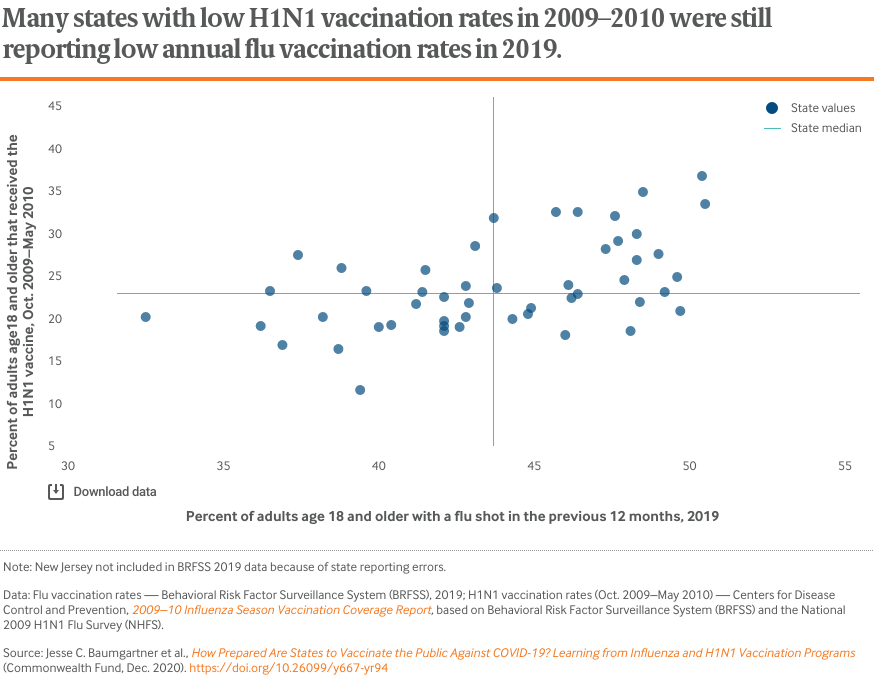
Although nearly a decade separates the two vaccination programs, and distribution of the vaccines differed, similarities exist in how rates varied from state to state. There is a modest but noticeable correlation between state vaccination rates for 2019 seasonal influenza and the 2009–2010 H1N1 virus. Many states with low flu vaccination rates, such as Florida, Georgia, Louisiana, Mississippi, and Texas, also reported below-average vaccination during the H1N1 pandemic.
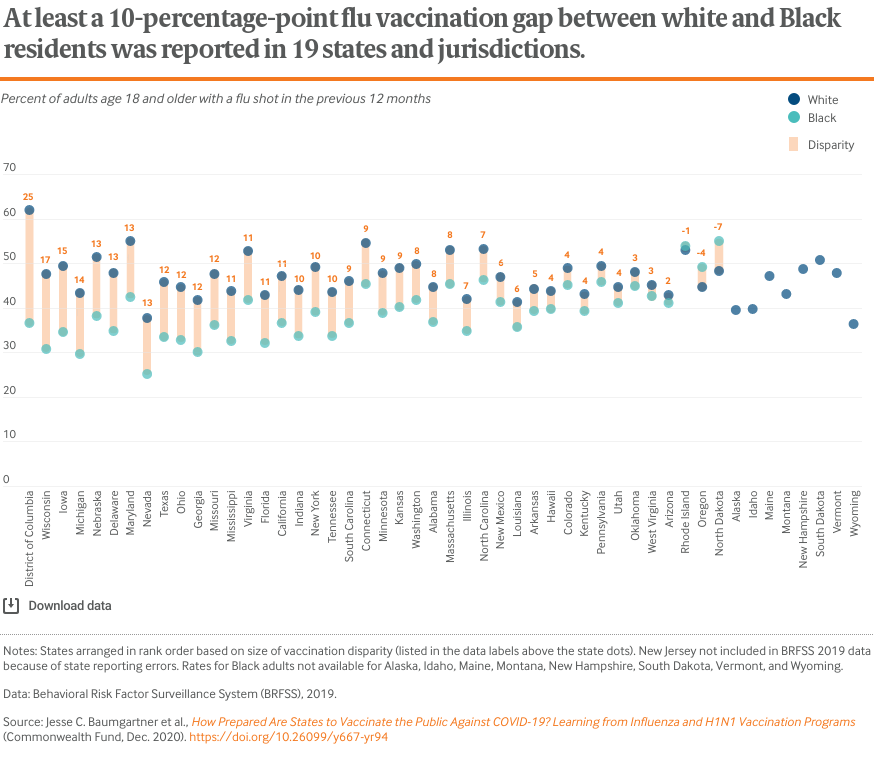
Annual influenza vaccination data reveal racial inequities across virtually every state in the country, with some states standing out much more than others. For example, Georgia, Maryland, and Texas, home to some of the nation’s largest Black communities, report some of the highest racial gaps in vaccination rates. Black Americans have contracted coronavirus and died from COVID-19 at disproportionately high rates.8
These disparities also occurred during the H1N1 vaccination effort (see box). To ensure that the most affected communities are adequately protected, states will need to acknowledge and address these and other inequities when developing distribution plans for COVID-19 vaccines once they come online.
Health Care Access and the Persistence of Racial and Ethnic Inequities: What National Vaccination Data Can Tell Us
National-level data can provide important insights into specific population challenges that states will face as they try to maximize uptake of a COVID-19 vaccine among all their residents.
Affordable access to care is a key prerequisite for vaccination. People who have health insurance, have a usual source of care, have no cost barriers, or have received a recent check-up were all much more likely to receive their annual flu shot (Table 2).
Individuals with chronic health conditions — probably because they interact with the health system more often than the average person — report some of the highest national vaccination rates, around 60 percent (still below the national target of 70%).
Age, income, and education are strongly associated with vaccine uptake. People age 65 and older were the only adult age group with flu vaccination rates well above 60 percent. This is encouraging, given the significant COVID-19 hospitalization and mortality risk associated with older age.
People with incomes above 400 percent of the federal poverty level (46.1%) and those with some level of college education (53.5%) were also much more likely to receive flu vaccinations.
Racial inequities in vaccination rates have persisted since H1N1 and have multiple causes. Nearly all communities of color were less likely to receive their annual flu vaccination shots in 2019, including many groups that have been severely affected by COVID-19 (Table 2). During the H1N1 pandemic, when states largely controlled allocation and initial supply was limited,9 racial inequities for Black adults were replicated across age groups and at-risk individuals (Table 3). This raises significant questions about the ability of states to equitably distribute and administer a COVID-19 vaccine.
Although vaccination is linked to coverage and financial barriers that reflect a long legacy of racial inequity (Table 2), flu and H1N1 studies also have found that Black and, in some cases, Latino respondents were more likely to voice concerns about the safety of vaccines and less likely to receive their vaccine from certain locations outside of a doctor’s office.10 This distrust stems in large part from institutional experiences with racism and unethical medical experimentation. Findings from recent surveys about a future COVID-19 vaccine show high levels of concern and hesitancy among Black respondents.11
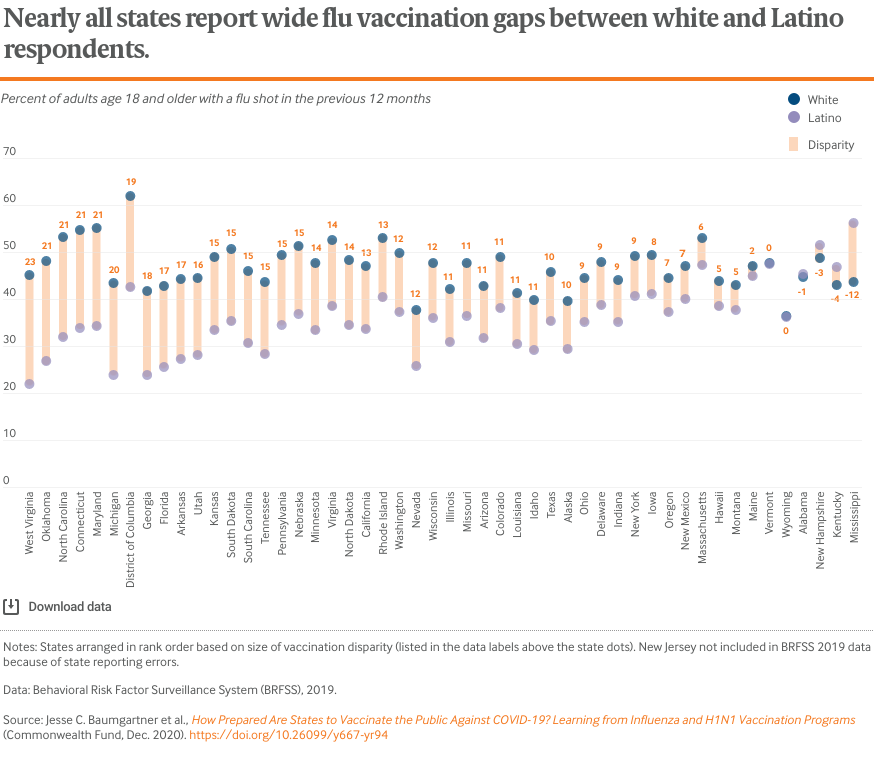
The flu vaccination gap for Latino adults, who already face greater barriers than other groups in obtaining insurance coverage and accessing care, is extremely wide within many states.12 Seventeen states have gaps with white adults of at least 15 percentage points, and in six of those states it is at least 20 points.
Among the five states with the largest Latino populations — California, Texas, Florida, New York, and Arizona — all but New York report vaccination differences exceeding 10 points.
Discussion
The COVID-19 pandemic continues to rage throughout the United States, hitting some states much harder than others.13 In addition to the time it takes for any infectious disease to spread, differences in population density, residents’ economic circumstances, travel patterns, state and local public health directives, and people’s adherence to practices like wearing face coverings and maintaining physical distance have contributed to these different state experiences.
The fast and variable spread of the novel coronavirus underscores the need for strong federal leadership to ensure fair allocation, rapid distribution, and wide administration of a future vaccine, particularly to high-risk groups. But the story of past adult vaccination programs is not encouraging. Even though data from the Centers for Disease Control and Prevention for the 2019–2020 flu season show improvement (and early 2020–2021 data have also been promising), no state comes close to achieving target adult vaccination rates.14 Even worse, COVID-19 cumulative case rates are higher in many states with poor vaccination rates.
A successful U.S. pandemic response will depend on strong federal planning, collaboration, and resources to support effective state responses.15 The federal plan for delivering vaccine supply to the states has not been released publicly and may be modified by the incoming Biden administration.16 The CDC recently collected initial state plans for allocating and distributing new COVID-19 vaccines.17 For effective distribution, state and local health departments will need to track and coordinate with a wide mix of private medical providers and publicly funded clinics that serve different communities. Additionally, they must help finance vaccination for the more than 35 million people in the United States who remain uninsured — disproportionately individuals with low income or people of color.18 With the right federal, state, and private company support, high and equitable vaccination rates are possible — as childhood vaccination programs suggest.
As the pandemic has already illustrated, the actions taken by state leaders can result in health burdens that are greater for some populations than for others. The toll has so far fallen disproportionately on essential workers, health care workers, the elderly, and others with an elevated risk of COVID-19 complications, as well as Black, Latino, American Indian, Alaska Native, and Native Hawaiian or Pacific Islander communities, among other groups. Tragically, as all aspects of the pandemic are increasingly politicized, states and providers also must overcome the growing public skepticism about the effectiveness and safety of vaccines, particularly among Black Americans, whose past experiences with racism in health care may have diminished their trust.19
How Government Can Respond
The federal government and the states have a number of options for overcoming these obstacles as they prepare to vaccinate the general population:
- Enhance the federal role in COVID-19 vaccination. The federal government typically purchases and distributes pandemic vaccine supply based on state-specific allocation requests, but current circumstances may demand more. A recent report from public health experts called for a coherent national distribution strategy in which more targeted state allocation decisions could be made at the federal level, particularly when early supply is limited.20 For now, the outgoing administration has indicated that initial state allocations will be based on total adult population, with states responsible for prioritization of who receives the vaccine first.21 A coordinated federal agency response would need to expand state funding to bolster vaccination capacity,22 standardize state distribution strategies, assist states by operating centralized storage and administration facilities, and sponsor and support state and local vaccine awareness campaigns (see below).
- Prioritize racial and ethnic equity in allocation. Reflecting wide-ranging racial and ethnic inequities within health, housing, and economic opportunity, communities of color have been hit hardest by COVID-19.23 Many of the disproportionately affected groups are ones less likely to get vaccinated. States can work with the CDC to design a vaccine allocation plan that accounts for these inequities by specifying protocols for prioritizing distribution to at-risk populations. The Advisory Committee on Immunization Practices (ACIP), which advises the CDC on allocation guidance that will be released to states, is already considering such strategies put forth by the National Academy of Sciences and other public health experts.24 States also can take steps to reduce financial barriers and other impediments to access as well as partner with community organizations to maximize vaccination rates.25
- Produce a robust media campaign. Given vaccine safety concerns, a strong information, education, and awareness campaign will be critical to building public trust and reaching target vaccination levels — particularly in partnership with those communities that are less likely to be vaccinated. States and local jurisdictions will use their own departments and resources to reach their residents, but a significant, visible, and consistent federal media campaign should underpin these efforts.
- Ensure the elimination of vaccine cost-sharing in public vaccination programs. Even though the federal government will have purchased an initial supply of the vaccine, cost-sharing for additional services, such as dose administration, may still vary by type of health insurance.26 Federal and state governments can eliminate cost-sharing through administrative actions within federal programs like Medicaid and Medicare. Experts have urged the U.S. Department of Health and Human Services to ensure complete coverage through coordinated federal action.27 Removing these costs will likely be critical to achieving high levels of vaccination and equity in participation.
Data Sources
Seasonal Influenza: Flu vaccination data came from our analysis of the CDC’s 2019 Behavioral Risk Factor Surveillance System (BRFSS), which asks whether adults received a flu shot in the past 12 months. Because responses were collected throughout 2019, this means the data used in this brief include responses from both the end of the 2018–2019 flu season and the start of the 2019–2020 flu season. The CDC also produces annual flu season estimates based on time-specific segments of two-year BRFSS samples, which are generally a few percentage points higher than the rates presented in this analysis (see the recent 2019–2020 flu season release28).
H1N1: Estimates for 2009–2010 H1N1 monovalent vaccination coverage came from CDC estimates based on BRFSS and the National 2009 H1N1 Flu Survey (NHFS). This reflects people who reported receiving the vaccination between October 2009 and May 2010.29
Acknowledgments
The authors thank David Blumenthal, Liz Fowler, Barry Scholl, Chris Hollander, Paul Frame, Jen Wilson, Sara Collins, Munira Gunja, and Gabriella Aboulafia, all of the Commonwealth Fund.
