COVID-19 vaccination has substantially mitigated the burden of COVID-19, preventing millions of deaths and hospitalizations since the first vaccines were rolled out in late 2020. As of early September 2022, updated bivalent boosters, or boosters that provide protection against both the original virus strain as well as the Omicron variant (and its subvariants like BA.4 and BA.5), have been authorized. These are available for anyone who has completed their primary vaccination series and has not received any additional doses for at least two months.
Boosters are a necessary part of COVID-19 mitigation because vaccine-induced and natural protection against disease have been shown to be transient. However, booster uptake in the United States has steadily declined since the initial wave of the Omicron variant, and federal financial support for vaccination campaigns has not been replenished, partly because of the perception that the pandemic is over. As of October 3, 68 percent of the total U.S. population has been vaccinated with a primary series, but fewer than half of fully vaccinated individuals have received a booster dose. Only 36 percent of people age 50 and older have received their second booster dose. The rates of vaccination, including boosters, declined to less than 100,000 doses administered daily by September 12. That number is beginning to increase as more people get updated booster shots, but dwindling federal funding for vaccination threatens to undermine any goal of high coverage.
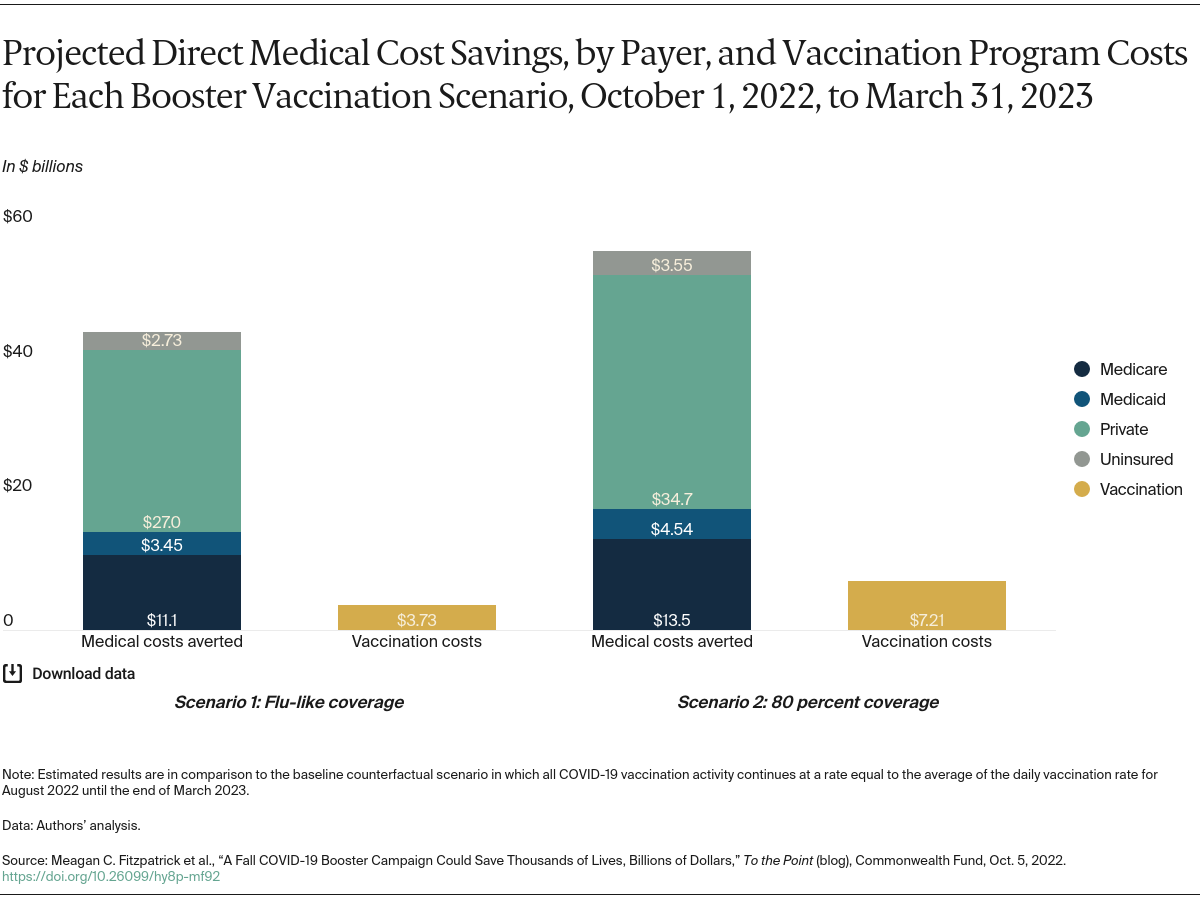
In this post we update our analysis from July and examine the potential impact of a surge in cases during the upcoming fall and winter. We evaluated the impact of accelerated delivery of booster doses on reducing hospitalizations, deaths, and direct medical costs. We conducted this analysis using a previously developed simulation model to estimate the impact of vaccination rates under different scenarios. To inform congressional decisions about federal spending, we identified the cost savings that would specifically accrue to the Medicare and Medicaid programs and compared these savings to anticipated expenditures on accelerated vaccination campaigns. (See “How We Conducted This Study” for further details.)
We examined three scenarios: a baseline scenario where daily vaccination rates remain unchanged and two vaccine campaign scenarios in which rates increased by the end of 2022. For the baseline scenario, we assumed that from September 2022, vaccination will continue at the same rate as the average in August (i.e., about 28 vaccine doses per 100,000 population per day) until the end of March 2023. In the two vaccine campaign scenarios, we simulated two different fall vaccination campaigns delivering booster doses to individuals age 5 and older at increased rates between October 1 and December 31, and at baseline rates for other times. (Following the operational guidelines of the Centers for Disease Control and Prevention (CDC) and in light of the Pfizer application regarding pediatric boosters, we considered those age 5 and older to be eligible if they had received the last dose of their primary series or booster dose at least four months prior.)
The two vaccine campaign scenarios where rates increase differ in the level of coverage achieved:
- In the first scenario, we estimated COVID booster uptake for the eligible population based on age-specific influenza vaccination coverage in 2020–2021 by the end of 2022
- In the second, we assume 80 percent of eligible individuals age 5 and older receive their booster dose by the end of 2022.
We then determined the estimated impact of these potential campaigns by comparing the projected number of infections, hospitalizations, deaths, and direct medical costs from October 1 to March 31 to the baseline scenario. For this analysis, we stratified estimates of cost savings by payer (e.g., Medicare, Medicaid, commercial insurance companies).