Abstract
- Issue: Public and private payers in the United States negotiate prices with drug manufacturers but in a less structured manner than in Germany. This has led to higher prices, administrative burdens on physicians, and significant cost-sharing for patients in the United States, compared with Germany.
- Goals: Describe how Germany sets drug prices and identify lessons for the United States.
- Methods: Interviews with leaders in payer, policy, patient, and pharmaceutical industry organizations in Germany.
- Key Findings and Conclusion: Germany’s system, which uses centralized drug assessment and price negotiation for new drugs coupled with reference pricing for noninnovative drugs, has resulted in substantially lower drug prices compared with the United States. Germany encourages manufacturers to moderate prices of innovative drugs through positive incentives (such as immediate coverage and the ability to obtain full list price for the first year after launch) and through negative incentives (such as mandatory arbitration when price negotiations fail). In the United States, efforts to control drug spending have centered on limiting patient access. U.S. drug prices could be reduced if Medicare and private payers standardized how they evaluate the clinical benefit of a drug, translate the benefit into a price, and resolve disagreements between negotiating parties.
Introduction
Recent debates over drug policy reform in the United States have often been marked by political rancor.1 That discord is notably absent in Germany, whose approach to setting drug prices is largely accepted by all stakeholders. While various groups in the country have ideas for incremental improvements, none dispute the legitimacy of Germany’s approach, which involves centralized pharmaceutical assessment and price negotiations.
Both Americans and Germans receive health care through competing, private health plans. Yet drug prices in Germany are substantially lower than in the U.S. Insurers in Germany impose no prior authorization requirements on physicians. Guidelines for appropriate prescribing are developed by physician associations, not by health insurers. And only nominal cost-sharing is required of German patients, who pay neither coinsurance nor deductibles. This contrasts with trends in insurance benefit design in the U.S., where patients increasingly are required to satisfy an annual deductible plus up to 33 percent coinsurance for their prescriptions.
This issue brief describes the institutional structure of the German pharmaceutical pricing system and its applicability to the U.S.
Germany’s Multipayer Health System
In Germany, there is no public insurance plan or option. All citizens and permanent residents have their health insurance benefits administered through private managed care plans. Most Germans obtain health insurance from one of 110 competing, nongovernmental “sickness funds,” with the premiums paid by employers and employees and with governmental subsidies for the unemployed and retired.2 These nonprofit health plans participate in the national system of employer-sponsored payment, risk adjustment, and centralized price negotiations with physicians, hospitals, and pharmaceutical manufacturers.
Approximately 10 percent of the population, mostly high-income individuals, opts for coverage through one of 48 indemnity insurers rather than through the sickness funds. The indemnity insurers are a mix of nonprofit and for-profit firms that can charge premiums according to the expected health risk of the enrollee, which sickness funds cannot do. But unlike the sickness funds, indemnity insurers do not benefit from subsidies for renouncing underwriting. The insurance system is managed by the Federal Joint Committee (G-BA), a private organization governed by the associations of sickness funds, physicians, hospitals, and patient advocates.3
A Self-Governed System for Setting Drug Prices
Germany’s institutional structure for drug pricing includes three main components:
- a scientifically focused but politically accountable entity that conducts health technology assessments for each new drug
- price negotiations between drug manufacturers and the association of health plans, and
- reference pricing for noninnovative drugs with therapeutically similar alternatives.4
Germany’s approach to setting drug prices is self-governed by insurers, providers, and patient advocacy groups, but it is subject to oversight by the federal Ministry of Health and, indirectly, by legislative committees at the state and federal levels. Elected officials are politically accountable to voters for the ease of access to drugs, to employers and employees for the premiums charged by the sickness funds, and to taxpayers for the subsidies needed to finance the gaps in the insurance system.
Drug price negotiations are conducted not by each insurer individually but rather by the national association of sickness funds, the GKV-SV. Collective negotiations permit payers to achieve a scale and leverage considerably larger than that available to any one insurer. Indemnity insurers do not participate in these negotiations but will pay the prices that emerge.
The negotiated drug price will be paid by all the sickness funds and indemnity insurers regardless of the volume each uses. The negotiations generate a discount that lowers the price for all payers and purposes, and not a rebate that varies across insurers based on their scale and volume of drug purchases. The new price is transparent to any entity willing to subscribe to the publicly maintained Lauer-Taxe database (originally developed so that pharmacies know which price to pay to drug distributors). Germany is the only nation for which negotiated net prices, and not merely manufacturer-established list prices, are transparent.
Both insurers and manufacturers face a strong reputational risk if they fail to agree on a price. More tangibly, failure to agree causes the drug to be referred to an independent arbitration board, which conducts its own analysis of the product and establishes its own price. The sickness funds and indemnity insurers must accept and pay the board’s price. The manufacturer can withdraw its product but thereby forfeits all sales in Europe’s largest market. Relatively few drugs are withdrawn by their manufacturers; of these, the overwhelming majority were found by G-BA to offer no incremental benefit compared to existing drugs.5
Sometimes manufacturers withdraw drugs evaluated by the G-BA as offering only moderate incremental benefit over a low-priced comparator because the resulting net price could adversely affect their pricing strategy in other nations. Many nations use external reference pricing as one factor when setting prices, and a low price negotiated in the largest and wealthiest European nation would serve as a ceiling to prices elsewhere, except in the U.S.6
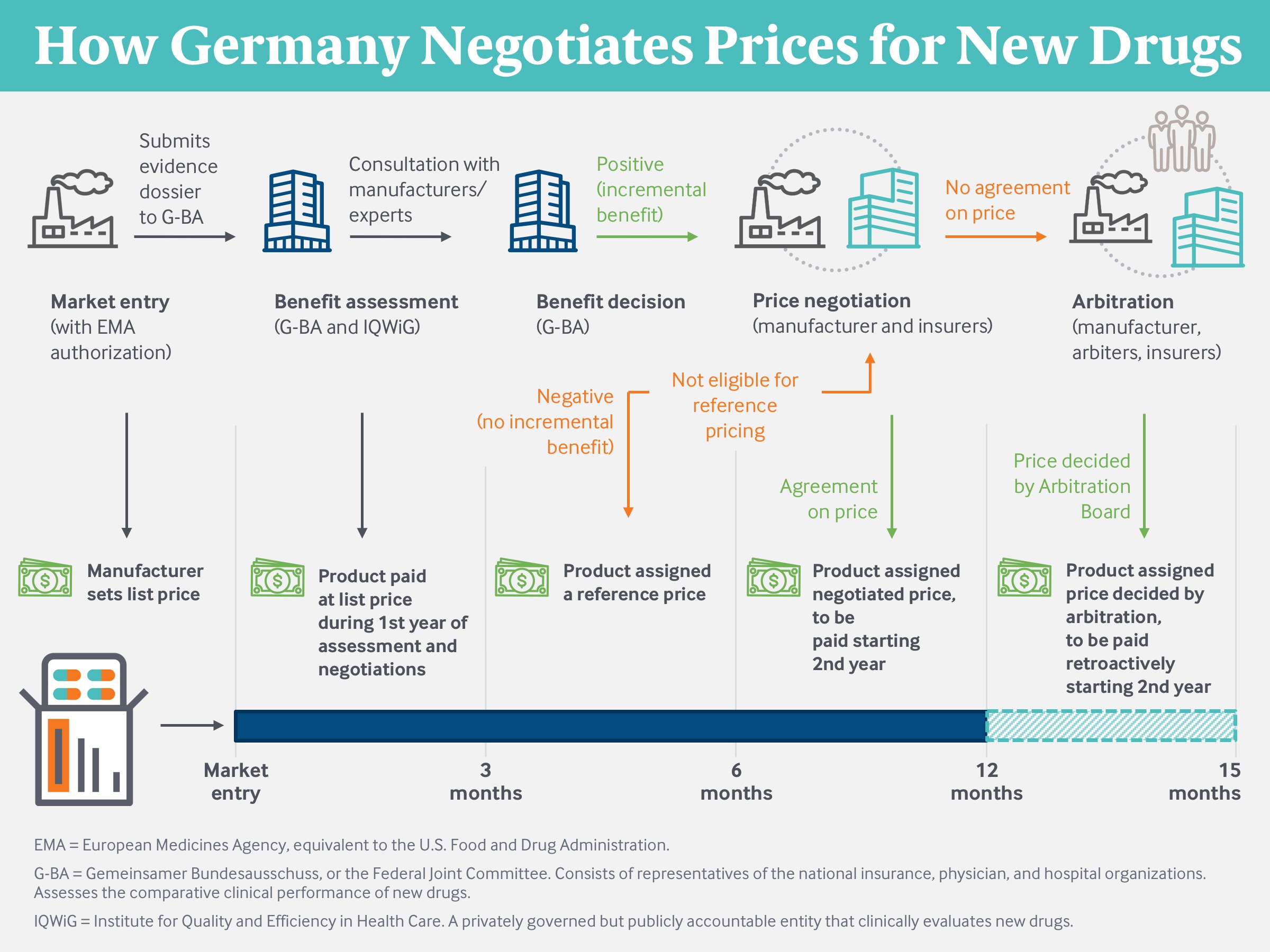
In Germany, a manufacturer is prohibited from unilaterally raising its price after the initial negotiations.7 A price only changes if the drug undergoes a new clinical evaluation by the G-BA, typically when new evidence of safety or efficacy is presented, and the GKV-SV negotiates a new price based on that evaluation. This contrasts with the U.S. system, where manufacturers routinely increase prices each year after launch, rarely with the support of new clinical evidence.8
The drug prices set through these combined approaches are significantly lower than those in the U.S., and the gap appears to be widening over time. U.S. net prices for the most expensive drugs are up to four times higher than their German equivalents.9 The Office of the Assistant Secretary for Planning and Evaluation (ASPE) estimates that U.S. prices for infused drugs covered under Medicare Part B are 80 percent higher than those in 16 comparable nations, including Germany.10 A recent study reported that net prices for infused drugs in the U.S. were 30 percent higher than those in Germany in 2006 and had risen to 60 percent higher in 2018.11
Implications for the Reform Efforts in the United States
Evaluation of Benefit
Historically, U.S. stakeholders have shied away from centralized price-setting for drugs and other health care technologies. Currently, the clinical benefits of new therapies are assessed, implicitly if not explicitly, by each insurer individually. It is unlikely that the U.S. will authorize a single entity to conduct health technology assessments for all public and private payers. However, each payer could be required to use a formal and evidence-based process, conducted internally or by an independent entity, as the basis for its coverage and reimbursement decisions.
The U.S. has a quasi-public entity in the Patient-Centered Outcomes Research Institute (PCORI), which conducts and commissions comparative clinical assessments. Currently, PCORI’s assessments cannot be used to determine insurance coverage or set prices. The private, nonprofit Institute for Clinical and Economic Review (ICER) conducts clinical and cost-effectiveness studies and recommends price benchmarks, which are currently used on a voluntary basis by payers such as the U.S. Department of Veterans Affairs, Express Scripts, and CVS Caremark.12
Utilization Management
The transparent and evidence-based manner by which drugs are evaluated in Germany has implications not merely for drug evaluation in the United States, but also for the manner in which U.S. payers are permitted to manage utilization. Currently, insurers and pharmacy benefit managers (PBMs) restrict physician and patient access to drugs through formulary exclusion, prior authorization, and cost-sharing. In some cases, these utilization management initiatives are developed in a transparent manner, but often they are not.13 Each insurer and PBM develops their own coverage and reimbursement rules at their own discretion, using a combination of published clinical literature or other evidence and financial considerations, including price negotiations. The U.S. health system could require utilization management to be based on comparative clinical evaluations in a manner transparent to physicians and patients.14
The German system does not allow sickness funds and indemnity insurers to use utilization management tools. All payers must cover all drugs approved by the G-BA. While they can conduct retrospective reviews to identify physicians whose prescribing patterns are outside the European Medicines Agency (EMA) and G-BA standards, they cannot require prior authorization to prescribe drugs. Consumer cost-sharing is modest and determined by statute, not by individual payers.
Reference Pricing
In Germany, innovative drugs that offer an incremental benefit over existing alternatives are rewarded with higher prices than those drugs that do not. These higher prices are proportional to their greater benefit over comparable products in the German market and in other European nations. These principles could be adopted by U.S. payers once a system of comparative clinical assessment was in commonwealthfund.org Issue Brief, January 2020 Drug Price Moderation in Germany: Lessons for U.S. Reform Efforts 5 place, since many insurers and PBMs already have the scale equivalent to the German (and other European) health insurance systems.
Manufacturers of noninnovative drugs are not allowed to charge more than their comparators and must compete for market share with lower prices, largely through the process of reference pricing. By way of contrast, the mainstay of U.S. pharmaceutical benefit design is the multitiered formulary. Under tiered formularies, the patient typically is responsible for the full price of expensive specialty drugs until the deductible is reached and then a percentage of the price until the annual, out-of-pocket maximum is reached. In contrast, reference pricing in Germany ensures there is always at least one drug in each therapeutic class available for nominal cost-sharing.
Reference pricing has been used to a limited extent by self-insured employers and labor unions in the U.S. and has resulted in product switching by patients and lower spending for sponsors. The savings have stemmed from switches from high-priced to low-priced drugs, rather than from actual price reductions, since the number of patients covered has been small.15 If adopted more broadly on the model of Medicare’s least costly alternative approach, reference pricing could generate savings not only through product switching but also through competitive price reductions.16
Conclusion
The German system of health technology assessment, price negotiations for innovative drugs, and reference pricing for noninnovative drugs has moderated spending with only a modest amount of governmental intervention. The contemporary mix of legislative proposals in the U.S. includes many features of the German approach, including price negotiations for innovative drugs and limits on postlaunch price increases. To achieve the results obtained by the German system in the U.S., these elements need to be combined with mechanisms to evaluate comparative clinical benefit, determine prices when negotiations fail, and obtain price discounts on noninnovative drugs.