Profile: In-Depth Look at an Initiative that Is Making a Difference
Washington State: An Integrated Approach to Evidence-Based Drug PurchasingSummary: Washington State's Health Care Authority, which coordinates the Prescription Drug Program for the state's Medicaid, public employee, and worker compensation programs, is using an integrated approach to value-based
pharmaceutical purchasing. The evidence-based drug review process involves a thorough analysis of quality and effectiveness before applying cost considerations. The process, which includes an evidence-based preferred drug list and supplemental rebates from pharmaceutical manufacturers, is producing savings of about $20 million each year to the state—over 5 percent of its Medicaid fee-for-service drug spending—and about $40 million in combined state-federal spending.
The Process
In June 2003, the Washington State legislature created the Prescription Drug Program
(PDP) to control state prescription drug costs while maintaining quality and to increase public awareness of safe and cost-effective drug use. The PDP is a joint effort of the Health Care Authority (HCA), the Department of Social and Health Services, and the Department of Labor and Industries. It is administered by HCA as a cross-agency entity that coordinates prescription drug purchasing for Medicaid fee-for-service, state public employee, and worker compensation programs.
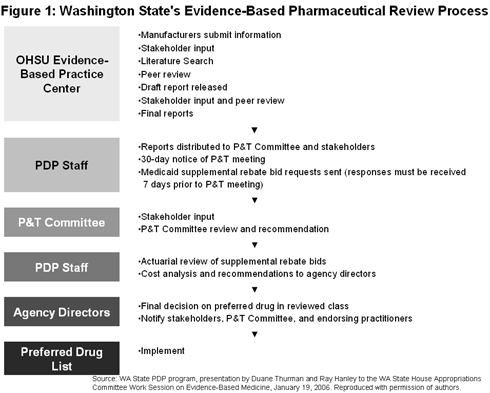
A key component of the PDP is the evidence-based review process, comprised of the following steps (also summarized in Figure 1, below):
- As a participant in the Drug Effectiveness Review Project coordinated at the Oregon Health Science University, HCA receives reports summarizing evidence on drug effectiveness for 26 drug classes that cover about half of pharmaceutical spending by the state.[1]
- A Pharmacy and Therapeutics (P&T) Committee made up of physicians, pharmacists, a registered nurse, and a physician's assistant reviews the reports and convense about every two months to evaluate the evidence for a certain drug class. Other stakeholders may also obtain the reports and participate in the meetings. The committee makes recommendations on drugs that are "equally safe and effective," without considering their cost.
- The state obtains bids for supplemental rebates from pharmaceutical manufacturers prior to the P&T meetings. After each P&T meeting, agency staff conduct an actuarial cost analysis of the drug or drugs recommended by the P&T committee for inclusion on the state's preferred drug list (PDL), using the supplemental rebate offers and other cost data to determine which drugs result in the lowest net cost to the state. In general, the committee recommends drugs they deem to be therapeutically equivalent, superior, or indicated for a particular condition or special population.
- Based on this cost analysis, the directors of each of the agencies make the final decision as to what drugs will be included on the PDL. This is a coordinated effort across the Medicaid fee-for-service program, the Uniform Medical Plan (UMP) (a coverage option for state employees and retirees, municipal workers, and retired teachers), and the state's Workers' Compensation Program.[2] In all, the evidence-based PDL applies to about 750,000 to 800,000 people. Program director Duane Thurman asserts that this process "tries to stay true to the evidence and P&T recommendations even if this results in a higher cost to the state." The agencies also use the reports on drug effectiveness to establish requirements for obtaining prior authorization for certain drugs.
- HCA notifies stakeholders, the P&T Committee, and "endorsing practitioners" about PDL and rule changes. An "endorsing practitioner" is any provider who has reviewed the state PDL and agrees to allow pharmacists to automatically exchange a non-preferred drug with a therapeutically equivalent preferred drug, unless he or she has specified to "dispense as written." [3] Those prescribers who are not endorsing practitioners may also request "dispense as written" for non-preferred drugs (that is, they are not bound by the PDL), but may be required to undergo a prior authorization process. [4] Thus, the reduced administrative burden of prescribing a non-preferred drug serves as an incentive for prescribers to become endorsing practitioners. A preliminary analysis of data from last year suggests that about 25 percent of Medicaid prescribers are endorsing practitioners, but they were responsible for about half of all Medicaid prescriptions.
The evidence-based pharmaceutical process has increased the market share of preferred drugs and reduced expenditures. For example, after adding ACE Inhibitors to the PDL in April 2004, there was an approximate 60 percent reduction in claims for non-preferred drugs in this class. Adherence to the PDL varies across drug classes, with an average rate of compliance of 80 percent.[5] Program officials are pleased with this outcome and are hoping that even more practitioners will become endorsing prescribers.
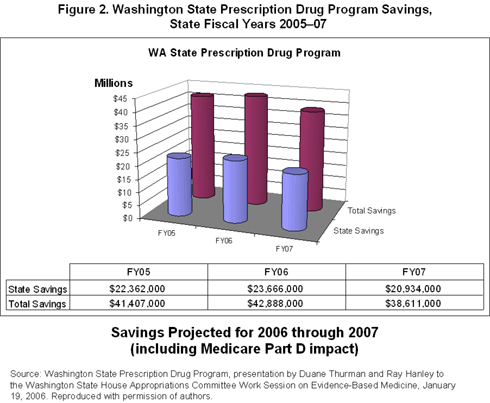
The cost savings are due to shifting market share away from non-preferred drugs and supplemental rebates from pharmaceutical manufacturers on drugs selected by the P&T committee as preferred. While some preferred drugs may be more expensive than others in their class, overall the preferred drugs are less costly to the state when factoring in the rebates. Across all agencies using the PDL, the state estimates combined savings of $20 to $23 million per year—about a 5 percent reduction in drug spending across the three state programs, and a $40 million per-year reduction in combined state/federal expenditures (Figure 2). [6]
Thurman stresses that Oregon's evidence-based drug program is a useful tool, but not the primary solution to rising costs. "Better is not always cheaper," he admits, "and quality takes precedence over cost considerations." Program officials expect that health outcomes will likely improve over time, although quantifying such an impact will be complex and require an analysis of long-term trends.
Challenges
While integration of the three state health programs is a hallmark and strength of Oregon's evidence-based drug program, it also has posed significant challenges. Medicaid, state employee health benefits, and worker's compensation have differing business models, prior authorization requirements, copayment structures, and relationships with pharmaceutical benefits managers. As a result, the PDL might save some state programs less than others. Further, collecting and synthesizing data from three programs have been difficult.
Overcoming pharmaceutical manufacturers' attempts to influence physicians and legislators, particularly when new drugs are rolled out, has also been challenging. HCA tries to educate legislators through presentations that emphasize the importance of the evidence-based approach. And, while mailings to practitioners have not been very effective, the agencies try to educate providers about the drug program and promote "buy in." They have conducted statewide training for pharmacists and provide ongoing training via the program Web site. In addition, Medicaid agency staff contacted the state's 200 heaviest prescribers to explain the program and the administrative advantages of becoming an "endorsing practitioner." Finally, the agencies work with the state medical association and local medical societies to distribute information and updates about the program.
HCA has faced a trade-off between thoroughness and timeliness. With each drug class review taking about 18 months to complete, the newest drugs entering the market are often not included in the PDL. HCA is addressing this through shorter "update reviews" on previously reviewed drug classes—enabling it to respond more quickly to new drugs without sacrificing the integrity of the process. Another typical challenge associated with PDLs is to avoid negative side effects that might be associated with therapeutic equivalents.
Next Steps
HCA will continue to monitor the evidence-based drug program and learn as it moves forward. Steps it plans for the near future include:
- evaluating the impact on the PDL of changes related to the dual eligible population as Medicare Part D is fully implemented;
- tracking endorsing and non-endorsing practitioner prescription patterns and implementing interventions to promote adherence to the PDL; and
- promoting preferred drug use through continued education to consumers and providers about the cost-effectiveness and efficacy of PDL drugs.
Washington's program is succeeding in part because it is an integrated, cross-agency process rather than just part of Medicaid, where it might be viewed as simply a cost-cutting effort. Also, Thurman stresses the need to keep the process transparent. Open public meetings help reduce distrust that certain stakeholders are "gaming" the process. They also cut through some of the mysteries around the value of certain drugs, helping purchasers and consumers understand what they are buying with their benefit coverage for pharmaceuticals.
References
[1] Eighteen classes have been reviewed to date and an additional eight drug classes are scheduled for review by January 2007. Although there are many more drug classes covered by each agency's program, these 26 classes account for the majority of drug spending.
[2] Fee-for-service Medicaid enrollees comprise about half of all Medicaid beneficiaries and include primarily disabled and elderly individuals. These beneficiaries are responsible for the majority of the state's drug spending. The PDL does not apply to Medicaid enrollees in managed care (primarily children and families).
[3] Also exempted are prescriptions for "refills" of certain drugs specified by statute; in these situations the pharmacist will dispense the non-preferred drug as prescribed.
[4] Prescriptions written by non-endorsing practitioners for drugs included on the PDL (except for refills of certain drugs specified by statute) and all prescriptions written for drugs that have not been through the evidence-based review process are filled according to the individual agency's pharmacy benefit structure.
[5] There is variation in compliance across drug classes, depending on the complexity of the therapeutic indications. The program administrators are currently collecting and examining these data.
[6] When drug spending for individuals who are dually eligible for Medicaid and Medicare is fully transferred to Medicare Part D, the savings to the state will decline somewhat, as overall pharmaceutical costs to the state decline.
|